Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
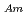 ) and Cm (
) and Cm (
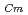 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
 (pyridine-dicarboxy-amide)
(pyridine-dicarboxy-amide) complexes
complexesDobler, M.; Hirata, Masaru; Tachimori, Shoichi
Physical Chemistry Chemical Physics, 2003(5), p.2499 - 2504, 2003/05
no abstracts in English
Suzuki, Hideya*; Naganawa, Hirochika; Tachimori, Shoichi
Solvent Extraction and Ion Exchange, 21(4), p.527 - 546, 2003/03
Times Cited Count:12 Percentile:45.03(Chemistry, Multidisciplinary)no abstracts in English
Sasaki, Yuji; Tachimori, Shoichi
Solvent Extraction and Ion Exchange, 20(1), p.21 - 34, 2002/01
Times Cited Count:186 Percentile:95.52(Chemistry, Multidisciplinary)no abstracts in English
Tachimori, Shoichi
Nihon Genshiryoku Gakkai-Shi, 42(11), p.1124 - 1129, 2000/11
Times Cited Count:1 Percentile:81.71(Nuclear Science & Technology)no abstracts in English
Naganawa, Hirochika; Suzuki, Hideya; Tachimori, Shoichi; Nasu, Akinobu*; Sekine, Tatsuya*
Bulletin of the Chemical Society of Japan, 73(3), p.623 - 630, 2000/03
Times Cited Count:6 Percentile:35.36(Chemistry, Multidisciplinary)no abstracts in English
Sasaki, Yuji
JAERI-Review 98-021, 198 Pages, 1998/11
no abstracts in English
Sasaki, Yuji
Bunseki Kagaku, 46(3), p.181 - 186, 1997/00
no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Journal of Radioanalytical and Nuclear Chemistry, 207(2), p.383 - 394, 1996/07
Times Cited Count:53 Percentile:96.15(Chemistry, Analytical)no abstracts in English
Sasaki, Yuji; Choppin, G. R.*
Analytical Sciences, 12(2), p.225 - 230, 1996/04
Times Cited Count:175 Percentile:98.64(Chemistry, Analytical)no abstracts in English
;
PNC TN8420 95-002, 140 Pages, 1995/01
In the field of uranium ore refining and spent fuel reprocessing, a lot of extractants capable of recovering actinides have been innovated, and some of which have been developed in actual industrial scale processes. Extractability against trivalent actinides on each extractants were evaluated in this report. And as for the compounds which showed excellent extractability for Americium, their extraction characteristics for trivalent actinides, such as extraction behavior, mechanism, ligand structure and conformation of complexes, were evaluated, and filed as data-base. Based on these works, some of extractant were newly identified as candidate to apply Americium recovering. The most appropriate compounds for trivalent actinide separation can be generally found in the neutral bidentate organic compound groups: Especially, some derivatives belongs to Diphosphineoxide, Carbamoyl-methylphosphine oxide and Propandiamide were superior to give sufficiently excellent property even in higher nitric acid(1 3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.
3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.
Sasaki, Yuji; Kitatsuji, Yoshihiro; Ban, Yasutoshi; Morita, Keisuke; Sugo, Yumi*; Saeki, Morihisa*
no journal, ,
Amidic and diamidic extractants, which can be gasified by combustion, have the strong affinity with actinides. One of the advantages to use these extractants is to synthesize easily, many kinds of structures can be applied for actinide extraction. It is well-known that the compounds used up to now have high distribution ratios of actinides from nitric acid, e.g., monoamide (for U and Pu), malonamide (for minor actinides), and diglycolamide (for minor actinides). As one of important researches to approach into the development of the best extractant, we compare the actinide extractabilities by the amide compounds with the different structures, especially different central frames. The purpose of this work is to investigate their extractability and to find the excellent structures among amide compounds. In the presentation, we will show not only results of extraction of actinides but also alkali, alkaline earth, group 3, 4 and 5, and discuss with their structures.
Tsutsui, Nao; Ban, Yasutoshi; Ishii, Sho*; Ito, Sayumi*; Suzuki, Hideya; Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*
no journal, ,
no abstracts in English
Sasaki, Yuji; Matsumiya, Masahiko*
no journal, ,
The separation of Lanthanides and actinides is noticed for not only atomic energy field but also general industry. These separation methods are requested on the points of Dy from Nd magnet and reductant of heat source and neutron emitter of Cm from Am. In the presentation, the results of separation of Dy and Nd by counter-current multi-step extraction will be mentioned.
Tsutsui, Nao; Nakase, Masahiko*; Ito, Sayumi*; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
To separate minor acitinides from lanthanides in nitric acid, we focused on 1,10-phenanthroline-2,9-diamides (PTDAs) which consist of both soft and hard donor atoms. The present study employed a PTDA having dodecyl groups in N atoms of amides groups, TDdPTDA, and its extraction property toward Am, Cm, and Eu was studied.
Tsutsui, Nao; Nakase, Masahiko*; Ito, Sayumi*; Ban, Yasutoshi; Matsumura, Tatsuro; Takeshita, Kenji*
no journal, ,
The extraction properties of three 1,10-phenanthroline-2,9-diamide derivatives (
 ,
,
 -diethly-
-diethly-
 ,
,
 -bis(4-ethylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Et(EtPh)PTDA),
-bis(4-ethylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Et(EtPh)PTDA), 
 ,
,
 -dihexyl-
-dihexyl-
 ,
, 
 -bis(4-hexylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Hex(HexPh)PTDA), and
-bis(4-hexylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Hex(HexPh)PTDA), and 
 ,
, 
 -didodecyl-
-didodecyl-
 ,
, 
 -bis(4-dodecylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Dd(DdPh)PTDA)) were studied for the separation of minor actinides (Am and Cm) and lanthanide (Eu) using single-stage batch methods. The results demonstrated that the PTDA derivatives extracted Am more efficiently than Eu or Cm. Furthermore, PTDA functionalized with short alkyl chains exhibited higher distribution ratios toward Eu, Am, and Cm when compared with those exhibited with longer chains. It was also revealed that the separation factor of Am with respect to Eu was relatively large, and that the alkyl chain length did not affect the separation factor when the same concentration of PTDA was used.
-bis(4-dodecylphenyl)-1,10-phenanthroline-2,9-dicarboxamide (Dd(DdPh)PTDA)) were studied for the separation of minor actinides (Am and Cm) and lanthanide (Eu) using single-stage batch methods. The results demonstrated that the PTDA derivatives extracted Am more efficiently than Eu or Cm. Furthermore, PTDA functionalized with short alkyl chains exhibited higher distribution ratios toward Eu, Am, and Cm when compared with those exhibited with longer chains. It was also revealed that the separation factor of Am with respect to Eu was relatively large, and that the alkyl chain length did not affect the separation factor when the same concentration of PTDA was used.